ABOUT US
History
The Cancer Research UK Prevention Trials Unit (CPTU) was established in 2006 to provide clinical trials unit (CTU) support to the Centre for Cancer Prevention (CCP) at the Wolfson Institute of Preventive Medicine (WIPH), part of Barts and The London School of Medicine and Dentistry (SMD), one of the three faculties of Queen Mary University of London.
In 2019, the CPTU moved to King’s College London, joining the Cancer Prevention Group within the Comprehensive Cancer Centre at the School of Cancer & Pharmaceutical Sciences, Faculty of Life Sciences and Medicine.
At the end of 2023, the CPTU returned to Queen Mary University of London to establish the Centre for Cancer Screening, Prevention and Early Diagnosis at the WIPH, relaunching the CPTU at QMUL.
Director of the CRUK CPTU, Professor Sasieni CBE is internationally recognised for his transformational work on the design and execution of clinical trials in cancer early detection and prevention. Professor Sasieni was awarded a CBE for services to cancer early detection and prevention in December 2024.
Research showcase
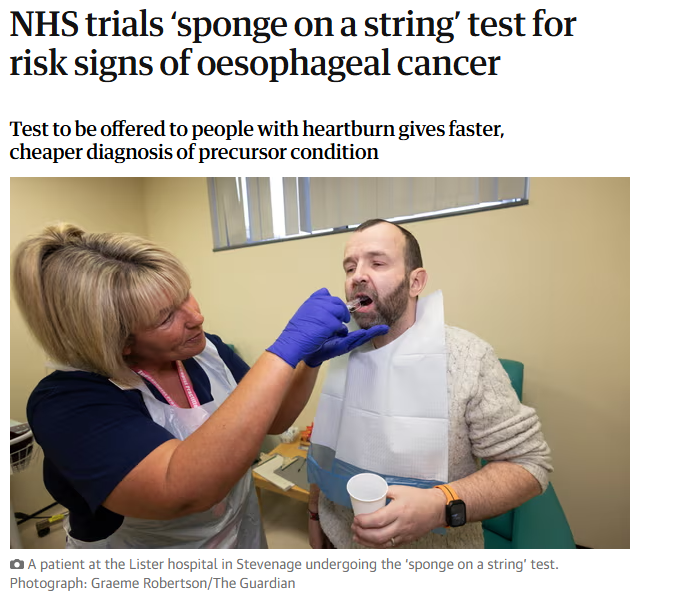
BEST4 Screening Trial
The BEST4 Screening trial opened to recruitment in November 2024. The trial aims to explore if the capsule sponge can prevent deaths from oesophageal cancer when offered as a screening test to people on long-term medication for heartburn – one of the most common Barrett's oesophagus symptoms. The trial aims to recruit 120,000 participantsa and and has recruited over 47,000 to date.
NHS-Galleri
Active follow-up for NHS-Galleri was completed in the summer of 2024. NHS-Galleri is a randomised controlled trial of the multi-cancer early detection blood test developed by GRAIL. With over 140,000 participants taking part, it represents the world’s largest research trial of a multi-cancer early detection blood test. Analysis is currently underway.